Gellan Gum Basic properties
Gellan gum is a polysaccharide produced extracellularly by a microorganism (Pseudomonas elodea); it is a straightchain heteropolysaccharide and is constituted of a repeating unit of four monosaccharide molecules, i.e.,glucose, glucuronic acid, glucose, and rhamnose, thus having one carboxyl group in the repeating unit (Fig. 1).
Though gellan gum has an acetyl group (C6) and a glyceryl group (C2) on its glucose residue immediately after production by fermentation (native gellan gum), this report will deal with deacylated gellan gum.
Method of dissolution
Gellan gum contains various kinds of inorganic ions, of which divalent cations prevent gellan gum from dissolving, so gellan gum is insoluble in cold water and has to be heated to 90 °C or higher to dissolve it; Sometimes in hard water it cannot be dissolved even at 90 °C or higher.
Gellan gum can be dissolved in hard water at room temperatures if divalent cations are sequestered by using a chelating agent. In this case, however, cations have to be added to the system in a greater amount than in the case of no chelating agent being used to form strong gel.
The following compounds are used as chelating agents in foods: trisodium citrate, sodium hexametaphosphate, tetrasodium pyrophosphate, trisodium phosphate, sodium polyphosphate, etc. In some cases a chelating agent and a divalent cation form a salt which is insoluble in water, so care has to be taken when clarity is necessary for the solution.
Thus the dissolution of gellan gum simply in cold water or hard water has to be carried out carefully,and its application to foods has to be made more carefully.
Food ingredients often include cations, proteins, insoluble solid matter, sugars, vitamins, carbohydrates,etc., and the constituents are very different from food to food; therefore, in order to make effective the addition of gellan gum to food, much consideration has to be given to the timing of addition and the properties of the solution to which gellan gum is to be added.
Method of gel formation
Unlike agar, gellan gum can’t form a gel simply by dissolving it under heat and then cooling the solution.Addition of a cation is necessary for the gelation of gellan gum. Particularly to obtain a heat-resistant gel, the addition of the divalent calcium cation is effective.
Also, gellan gum can be made to gel by lowering the pH of the solution. This is one of the features of gellan gum,but the gel so obtained has a tendency to have a lower clarity and a higher syneresis. For this reason, gelation of gellan gum by lowering the pH is not often used in food manufacturing, and the addition of inorganic ions is normally used.
Reactivity with cations
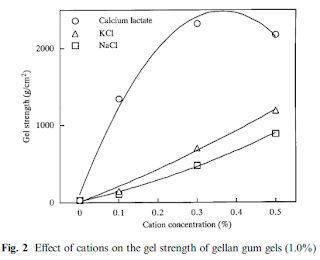
Though gellan gum can form a strong gel even from a dilute solution, the presence of cations is indispensable for its gelation. As shown in Fig. 2, the reactivity of gellan gum is much higher with the divalent calcium cation than with univalent cations, such as sodium, potassium, etc. Excessively high concentrations of the calcium ion, however, make the gel strength decrease.
Presumably, this is because the association of gellan gum molecules proceeds so far to the coagulation state that the stable three-dimensional network structure cannot be retained.
Setting and melting temperatures
Gellan gum is notable for its formation of heat-resistant gels. The gel melting temperature rises steeply with an increase in the calcium concentration, indicating that calcium will give heat resistance to the gel. The increase in the calcium concent, however,has little effect on the setting temperature.